Central Processing Sterilization Record Audit


How it works

Frequently asked questions
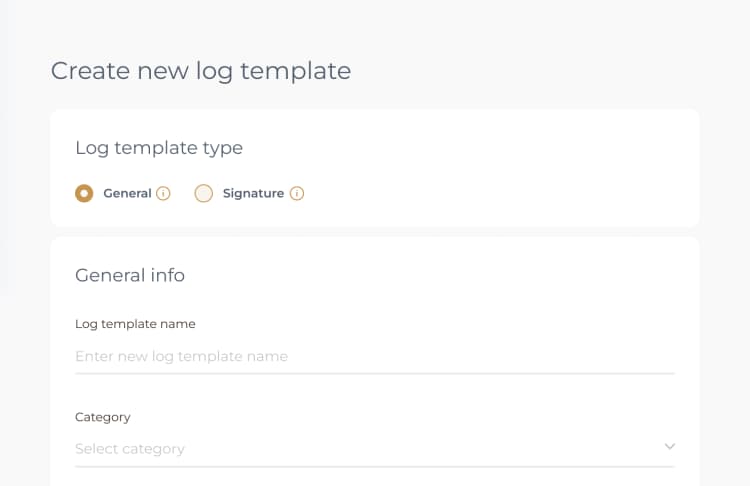
To start, you can access the monthly Central Processing Sterilization Record Audit Log template in PreferredMD. This can be done either by scanning the QR code that is provided or by navigating to the Facility Documents section. Once you have accessed the template, take the time to thoroughly review it. Make sure to pay close attention to the instructions provided and input all the necessary information accordingly.
Accessing the PreferredMD system on your smartphone via a web browser allows you to conveniently modify the monthly Central Processing Sterilization Record Audit Log. Simply navigate to the Facility Documents section within the system, where you can easily locate the template for the monthly Central Processing Sterilization Record Audit Log. From there, you can proceed to make any necessary edits to the log, ensuring it is up to date and accurate.
To perform the monthly Central Processing Sterilization Record Audit Log on an Android device, access the PreferredMD system through a web browser. Navigate to the Facility Documents section and locate the monthly Central Processing Sterilization Record Audit Log template. Follow the provided instructions to complete the log.
The completion of the monthly Central Processing Sterilization Record Audit Log is the responsibility of the assigned personnel who are in charge of sterilization procedures at the facility. This task should be carried out in accordance with the protocols and compliance requirements set by the facility.
The monthly Central Processing Sterilization Record Audit Log may need to be completed by individuals engaged in sterilization processes at the facility, which encompasses sterilization technicians, nurses, and medical staff. This log is an essential component of compliance procedures that ensures adherence to sterilization protocols and regulations.
Trained staff members who are responsible for sterilization processes in the facility usually complete the monthly Central Processing Sterilization Record Audit Log. Their main goal is to guarantee accuracy and adherence to regulatory standards throughout the process.
The monthly Central Processing Sterilization Record Audit Log serves as an official record that is utilized to meticulously monitor and track sterilization procedures carried out within a healthcare facility. This log plays a crucial role in providing a detailed summary of all sterilization activities, ensuring that the facility adheres to regulatory standards and prioritizes the safety of patients. By maintaining this comprehensive log, healthcare professionals can effectively manage and evaluate the sterilization processes, guaranteeing a high level of quality control and accountability.
The monthly Central Processing Sterilization Record Audit Log is a comprehensive document that provides a detailed account of various aspects related to sterilization procedures. It contains essential information such as the precise date and time when sterilization took place, the specific equipment that was used, the parameters of the sterilization cycle, and the batch or lot numbers associated with the process.
The consequences for failing to complete a monthly Central Processing Sterilization Record Audit Log on time can differ based on the policies of the facility and the regulatory requirements in place. These consequences may encompass disciplinary measures, financial penalties, or other repercussions that are clearly defined in the facility's compliance protocols.
The timeframe for finishing the monthly Central Processing Sterilization Record Audit Log is generally established based on the protocols and regulatory guidelines followed by the facility. Adhering to these predetermined timelines is crucial to maintain compliance with regulations and ensure the safety of patients.
The monthly Central Processing Sterilization Record Audit Log serves the crucial purpose of maintaining precise documentation regarding all sterilization procedures carried out within the facility. By doing so, it plays a vital role in ensuring that the facility remains compliant with regulatory standards, while also allowing for the tracking of equipment usage.
The monthly Central Processing Sterilization Record Audit Log utilizes a data logger, which can be either a device or software, to effectively track and monitor various sterilization parameters. These parameters include temperature, pressure, and cycle duration. By automatically recording this information, the data logger ensures that there is additional documentation and assurance regarding the effectiveness of the sterilization process.
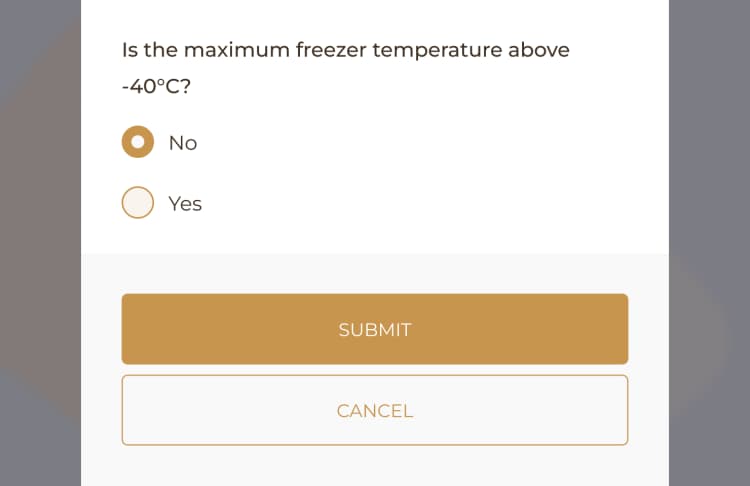
The monthly Central Processing Sterilization Record Audit Log checklist could consist of various tasks, including validating sterilization cycle parameters, ensuring equipment is functioning correctly, verifying proper loading and placement of items, and recording any deviations or incidents that occur throughout the sterilization process.
When conducting monthly Central Processing Sterilization Record Audit Log checks, it is essential to adhere to the appropriate guidelines. These guidelines are based on established protocols and regulatory requirements that are specific to the facility and the type of sterilization procedures being carried out. It is crucial to follow manufacturer instructions, industry standards, and best practices to ensure the accuracy and effectiveness of the audit log checks. By doing so, the facility can maintain a high level of quality control and ensure that all sterilization procedures are performed correctly and in accordance with the necessary guidelines.
The monthly Central Processing Sterilization Record Audit Log requires a range of equipment, including autoclaves, sterilizers, biological and chemical indicators, data loggers, and documentation tools, to ensure proper sterilization procedures
Accessing the monthly Central Processing Sterilization Record Audit Log in the PreferredMD system is a breeze. Simply open a web browser on any device connected to the internet, and you're good to go. Once you're in the system, head over to the Facility Documents section where you'll find the template waiting for you to fill out and complete.
To modify the monthly Central Processing Sterilization Record Audit Log on your Android device, you have the option to access the PreferredMD system via a web browser. All you need to do is go to the Facility Documents section and find the template for the monthly Central Processing Sterilization Record Audit Log. From there, you can easily make any necessary edits to the log.
Ensuring precise documentation of sterilization procedures, monitoring equipment usage, evaluating sterilization effectiveness, and adhering to regulatory standards are all crucial aspects of completing the monthly Central Processing Sterilization Record Audit Log. By diligently maintaining this log, healthcare facilities can prioritize patient safety and maintain a high standard of care.
An audit log for sterilization records is essential whenever sterilization procedures take place in the facility. This log must be filled out promptly following each sterilization cycle to guarantee precise documentation and adherence to regulatory standards.
To ensure compliance , it is crucial to carefully evaluate the accuracy of your monthly Central Processing Sterilization Record Log entries, the comprehensiveness of the information, and the prompt submission of the log. By paying close attention you can guarantee that all requirements are met and maintain the smooth functioning of operations.
PreferredMD makes compliance logging simple and paperless

![[object Object]](/_next/image?url=https%3A%2F%2Fpreferredmd.io%2Fimages%2Flog-template%2Flogs-dashboard.webp&w=750&q=75)
Get the
Open log templateRequest a demo and start your paperless journey
Schedule a demo