Shelf Life Audit Log


How it works

Frequently asked questions
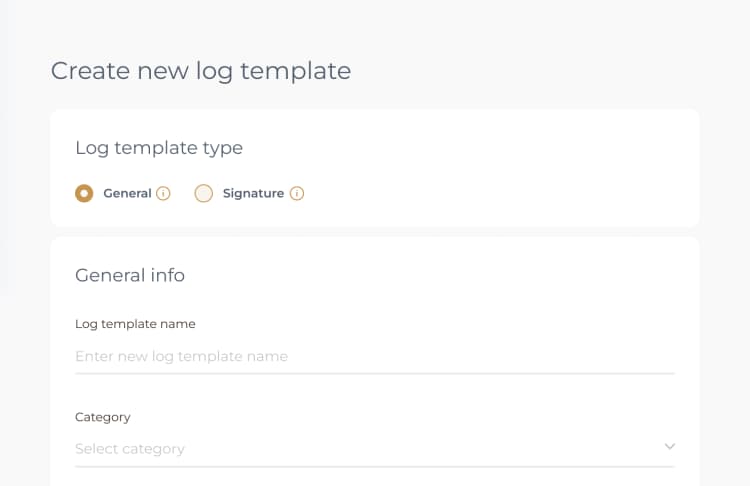
When completing the Shelf Life Audit Log template, you can begin by accessing the log in the PreferredMD system. This can be done by scanning the relevant QR code or navigating to the Facility Documents section. Once there, make sure to input all necessary details about the items being audited, ensuring that all fields are accurately filled out.
To edit a Shelf Life Audit Log using a smartphone, start by opening the PreferredMD app or website. Then, navigate to your Facility Documents and find the specific log you want to edit. Choose the edit option and you'll be able to update the required information directly on your device.
To complete the Shelf Life Audit Log using an Android device, open the PreferredMD platform on your Android device, navigate to the Facility Documents section, then locate the Shelf Life Audit Log, and Input all pertinent information about the shelf life of the items. Remember to save the log once you have entered all the necessary data.
Personnel in charge of inventory management and compliance within the facility, such as inventory managers or quality control officers, are required to complete the Shelf Life Audit Log.
A Shelf Life Audit Log is an essential tool for facilities that need to monitor and validate the expiration dates of their inventory items. It is crucial for maintaining compliance with regulatory standards and ensuring the safety and effectiveness of products.
Designated staff members, such as inventory managers, are responsible for completing this log. Their task is to monitor and record the expiration dates and conditions of stored items.
A Shelf Life Audit Log is a detailed record that helps keep track of the expiration dates and conditions of items in inventory. It ensures that the items are within their usable life span and meet the required quality standards.
The log must contain specific information including the names of the items, batch numbers, expiration dates, details about storage conditions, and any discrepancies or actions taken in relation to the shelf life of the items.
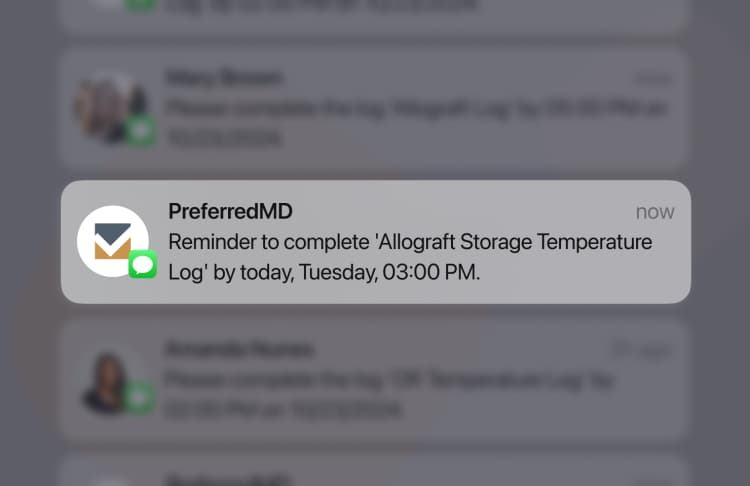
Please ensure that the Shelf Life Audit Log is completed on time to avoid potential non-compliance penalties. These penalties may include fines or other disciplinary actions, and their severity can vary based on regulatory requirements and internal facility policies.
The Shelf Life Audit Log must be completed by the deadline as specified by the facility’s compliance schedule or regulatory guidelines. Strict adherence to this deadline is crucial to avoid potential penalties.
The Shelf Life Audit Log serves the crucial function of verifying that all items in inventory are safe, functional, and still within their designated shelf life. This process is essential for upholding product quality and ensuring compliance with regulations.
A data logger is a sophisticated electronic device that is specifically designed to continuously record and store crucial information over a period of time.
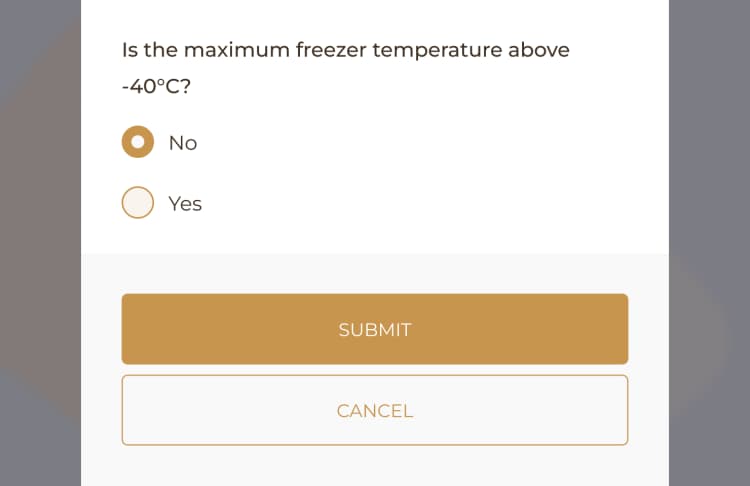
When conducting a Shelf Life Audit Log, it's important to meticulously review item names, batch numbers, expiration dates, and storage conditions. Any discrepancies or necessary actions to ensure inventory compliance should be carefully noted.
The process of conducting Shelf Life Audit Log checks entails thoroughly examining the expiration dates, storage conditions, and overall quality of products, while adhering to the facility’s compliance protocols and regulatory standards.
Essential equipment for managing our inventory includes specialized software to keep track of our stock, data loggers to monitor environmental conditions, barcode scanners for efficient item tracking, and a computer or mobile device to access and update the PreferredMD system.
You have the flexibility to fill out the Shelf Life Audit Log from any location with internet access. You can use a computer or mobile device to access the PreferredMD system and enter all the required details.
To edit a Shelf Life Audit Log on an Android device, you can access the PreferredMD system through your mobile browser or app. Once you are in the system, locate the log and select the option to edit the entries.
It is essential to diligently maintain the Shelf Life Audit Log to guarantee that all inventory items are utilized within their designated shelf life. This is crucial for upholding quality standards, ensuring safety, and complying with regulations.
A Shelf Life Audit Log is an essential tool for monitoring and recording the expiration dates and storage conditions of items, especially in environments where strict regulations apply.
When utilizing a Shelf Life Audit Log, it's important to carefully assess the precision of the documented data, conformance with regulatory standards, the state of storage conditions, and regular updates to guarantee adherence and quality assurance.
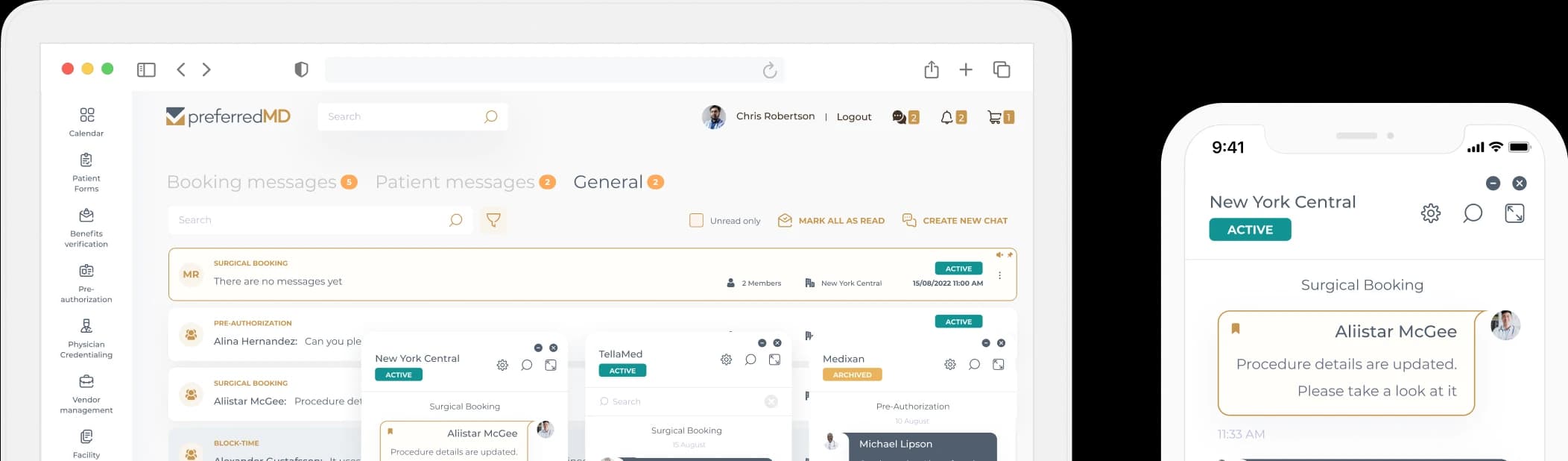
PreferredMD makes compliance logging simple and paperless

![[object Object]](/_next/image?url=https%3A%2F%2Fpreferredmd.io%2Fimages%2Flog-template%2Flogs-dashboard.webp&w=750&q=75)
Get the
Open log templateRequest a demo and start your paperless journey
Schedule a demo