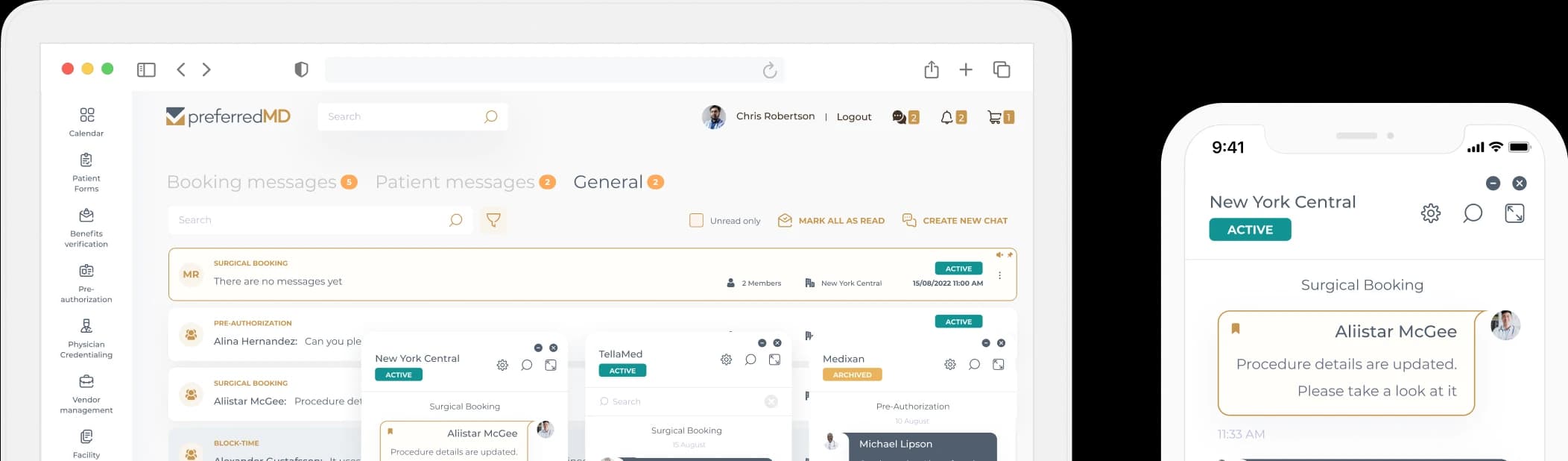
Pathology Audit Log


How it works

Frequently asked questions
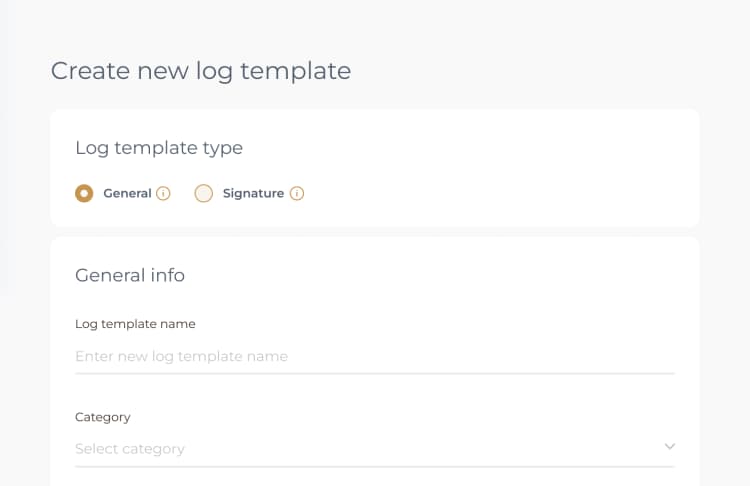
To complete the Pathology Audit Log template, you will need to access it within the PreferredMD system. This can be achieved by either scanning the relevant QR code or by navigating through the Facility Documents section. Once you have accessed the template, please ensure to enter all required information accurately into the log template, making sure that all fields are properly filled.
To edit a Pathology Audit Log on a smartphone, simply open the PreferredMD app and navigate to the Facility Documents section. From there, locate the specific audit that requires editing and make the required changes directly within the app interface.
To conduct a Pathology Audit on your Android device, first open the PreferredMD app. Then, go to the Facility Documents section, where you can find the Pathology Audit Log template. Fill in all the necessary information and make sure to save your entries.
In most healthcare facilities, the task of completing Pathology Audit Log is usually assigned to the pathology staff or compliance officers responsible for ensuring that the facility adheres to the necessary standards and regulations.
Healthcare facilities, such as hospitals and clinics that manage pathology samples, must conduct Pathology Audit Log to guarantee adherence to regulations and maintain precise documentation.
Pathology audits are typically conducted by staff members in the pathology department who are responsible for ensuring compliance and quality control.
A Pathology Audit is a comprehensive and methodical assessment of pathology procedures, documentation, and machinery to verify adherence to regulatory requirements and uphold the delivery of exceptional patient care.
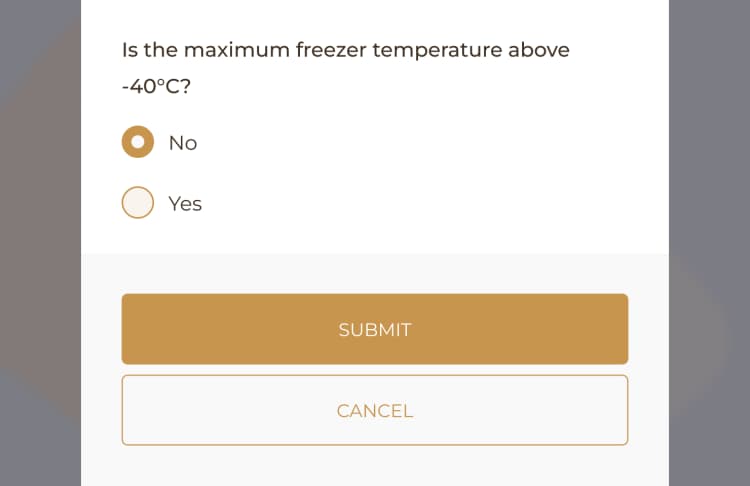
A comprehensive Pathology Audit should encompass detailed data including but not limited to sample identification, arrival time, storage conditions, processing duration, test results, quality control checks for equipment, any discrepancies or anomalies from standard operating procedures, and any corrective actions taken.
The consequences for failing to complete a Pathology Audit on time can differ, but they might involve financial penalties, disciplinary actions from regulatory authorities, or even the revocation of accreditation, depending on the specific regulations and guidelines set forth by the governing bodies.
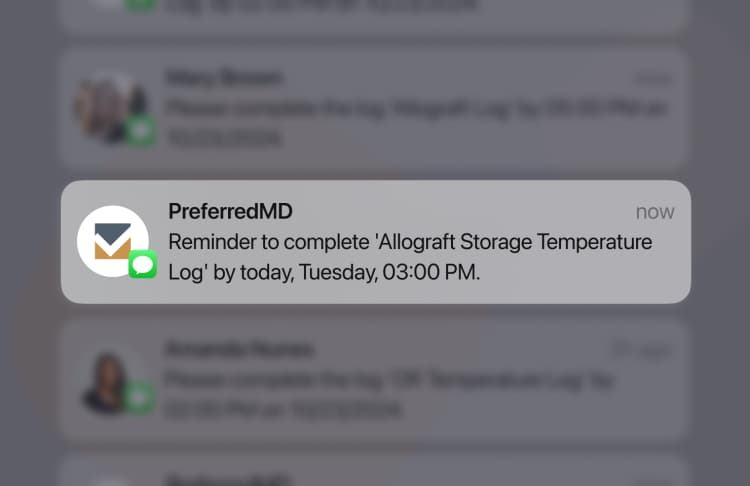
The deadlines for completing Pathology Audit Log are usually determined by regulatory agencies and facility guidelines. These deadlines often require monthly or quarterly submissions on a regular basis.
The deadlines for completing Pathology Audit Log are usually determined by regulatory agencies and facility guidelines. These deadlines often require monthly or quarterly submissions on a regular basis.
A data logger for Pathology Audit Log is a sophisticated electronic tool designed to automatically capture and store data on environmental conditions critical to the proper preservation of pathology samples. This includes monitoring and recording temperature and humidity levels to guarantee that the samples are maintained under ideal conditions.
In order to ensure the quality of Pathology Audit Log, it's important to thoroughly check and verify the integrity of the samples, meticulously review and confirm proper documentation, meticulously verify and validate equipment calibration, and rigorously ensure that all procedures strictly adhere to regulatory standards.
The proper procedures for conducting Pathology Audit Log require adhering to established protocols for handling samples, maintaining detailed documentation, ensuring equipment is well-maintained, and promptly reporting any irregularities or discrepancies.
Pathology Audit Log require specific equipment to ensure precise testing and meticulous record-keeping. This includes microscopes, calibration tools, data loggers, and other essential laboratory instruments.
You have the option to complete a Pathology Audit Log either through the online portal on the PreferredMD system or by utilizing the dedicated application on your smartphone or tablet.
To edit a Pathology Audit Log on an Android device, you can open the PreferredMD app, find the specific audit you want to modify, and then proceed to make the required changes.
Ensuring that Pathology Audit Log are completed is essential because it guarantees that the processes within pathology adhere to regulatory standards. This is crucial for upholding patient safety and maintaining the integrity of the testing procedures.
Regular Pathology Audit Log are crucial and should be conducted on a monthly or quarterly basis. They should also be carried out whenever there are significant changes in processes or equipment that could impact compliance and quality.
When utilizing a Pathology Audit Log, it is important to prioritize precision in data entry, compliance with regulatory standards, timely execution, and comprehensive documentation of each stage and discoveries.
PreferredMD makes compliance logging simple and paperless

![[object Object]](/_next/image?url=https%3A%2F%2Fpreferredmd.io%2Fimages%2Flog-template%2Flogs-dashboard.webp&w=750&q=75)
Get the
Open log templateRequest a demo and start your paperless journey
Schedule a demo